

Can I get a false positive from the BinaxNOW COVID Antigen Self Test?įalse positives and false negatives are possible with all COVID-19 tests.Ībbott recommends testing twice over 3 days, waiting 24–48 hours between each test.
Lower prevalent areas had less chance of identifying those with the virus. Are COVID-19 self-tests reliable?Ī 2021 study states the rapid antigen test can accurately identify people who are likely to have the SARS-CoV-2 infection, especially in the setting of high prevalence. Here we answer some common questions about the BinaxNOW COVID-19 Antigen Self Test. Those who left positive reviews state that the test is accurate and easy to use.Ĭustomers who left more negative reviews mention product seals broken on arrival, expired tests, and missing components. However, customers on Amazon give the test an average of 4.7 out of 5 stars. Most of the reviews focus on other products the company offers rather than the BinaxNOW COVID-19 Antigen Self Test. The company has closed 63 complaints in the last 12 months.Ībbott has an average customer rating of 1.4 out of 5 stars on Trustpilot. Jude settled with the Civil Division and the District of Maryland for allegedly failing to disclose serious adverse effects regarding the company’s implantable defibrillators.Ībbott has an average customer rating of 1.05 out of 5 stars on the BBB. Department of Justice for alleged violations of the False Claims Act.Īdditionally, St. Two of Abbott’s acquisitions - Alere Inc. The company has been manufacturing the BinaxNOW COVID-19 Antigen Self Test since August 2020.Ībbott does not hold accreditation with the Better Business Bureau (BBB), and the organization does not give Abbott a grade. All other test results - such as a combination of a pink and a blue line, one blue line, or no lines - represent faulty results.Ībbott is a healthcare and medical device company and the owner and manufacturer of BinaxNOW. Negative test results will only show one pink line in the control section. If a person’s test is positive, two pink or purple lines appear in the control and sample section. Peel the adhesive off of the strip on the right-hand side of the card and close the left side over the right, pressing firmly on the edge to seal it closed.Turn the swab clockwise three times and leave the swab inside the card.Insert the swab into the bottom hole of the test card and firmly push the swab until it is visible in the top hole.Repeat with the second nostril using the same swab.Insert the swab into one nostril, around half an inch inside, and rotate the swab against the nostril walls five times for 15 seconds.Open the swab packet from the end with the stick, making sure to avoid touching the cotton swab.Remove the dropper bottle cap, and without touching the dropper to the card, squeeze six drops into the top hole of the card.Open the test card out and lay it flat without touching the test strip.Unwrap the test card and place it on a flat surface for the duration of the test, ensuring there is a blue line in the test window.Adults can perform the test on children who are at least 2 years old.Ībbott also provides an instruction video in English and Spanish on its website. The company also states that this test is suitable for people aged 15 years and older.

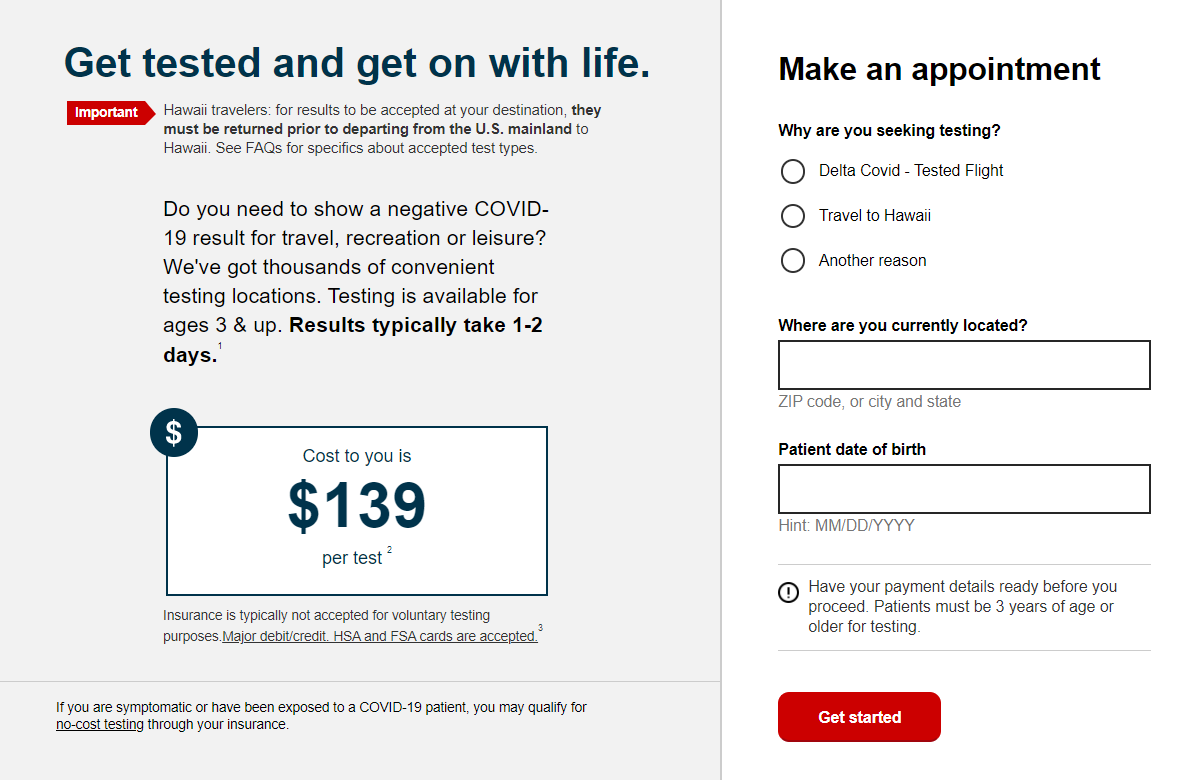
People with or without symptoms should test twice if negative, ideally 48 hours after the initial test. Not available in all locations.Taking the BinaxNOW COVID-19 Antigen Self TestĪbbott states that people should take the BinaxNOW COVID-19 Antigen Self Test within 7 days of symptom onset. In addition, access to additional health and safety solutions such as contact tracing, symptom checker and thermal scanner may be through third parties. Services described would be provided by CVS Pharmacy, Inc., or a subsidiary, including but not limited to MinuteClinic, LLC and its subsidiaries and managed entities. This is not intended to be a complete description of the terms and conditions of the Return Ready program. Competent legal counsel should be consulted. You may need to consider various federal, state and local laws, regulations, or other mandates when developing your return to worksite plans. The COVID-19 pandemic has created an unprecedented and still evolving legal landscape for organizations. Institutions should consider these factors in developing their return to workplace policies and procedures. Further, available testing procedures may produce false negative or false positive results due to a variety of factors. COVID‑19 testing does not eliminate the risk of transmission of SARS-CoV-2 or Coronavirus Disease 2019.


 0 kommentar(er)
0 kommentar(er)
